![]()
| For the past ten years, I have been working on the artificial pollination of stapeliads. This work has been carried out with a large collection of plants grown in my greenhouse in Flemington, New Jersey. Many obstacles associated with the growing of these plants away from their desert habitat have had to be overcome. Unfortunately, some problems of disease control remain unsolved, resulting in the loss of material which has been successfully pollinated. It is felt that at this time sufficient experience has been gained to report on my observations and the methods which I am currently using. Approximately a thousand pollinations have been carried out in each flowering season. Although I have had scattered blooms throughout the year, the peak flowering in this region occurs between late spring and late fall. Some species, such as Piaranthus, seem to limit their bloom to the fall while others flower sporadically throughout the blooming period. There are many variations of size and form in the blooms of the stapelias ranging from the huge flowers of Stapelia gigantea to the tiny flowers of Echidnopsis and Rhytidocaulon. All stapeliad flowers show variations from a basic pattern that has been well described using Orbea variegata as a model. |
| |
|
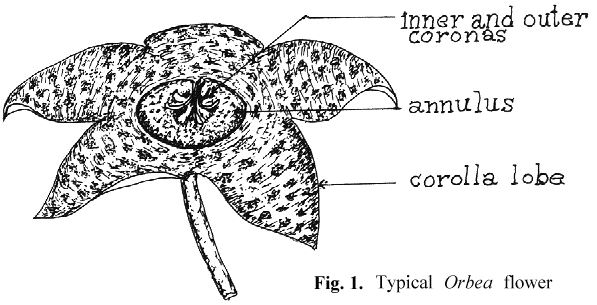
An appreciation of the technique for artificial pollination requires an understanding of the basic structure of the stapeliad flower and its pollination biology. Unlike the flowers of most higher plants which are pollinated by the transfer of granular pollen grains from the anther to the pistil, pollination in the stapeliads involves a highly specialized structure, the pollinarium.
To properly discuss the pollinarium requires an acceptance of terms for the description of its parts and of the rest of the stapeliad flower. There seems to have been general agreement in regard to the naming of the major parts of the bloom (see Fig. 1). On the other hand, the pollinarium has been given varying names for its parts. Newton 1 has attempted to resolve some of these differences by the use of the term "bipollinarium." This term does not appear to have been accepted.2,3 His paper also reviews a number of the earlier papers on pollinarium terminology. Since the pollinarium is the structure with which much ofthis paper will be dealing, it is necessary to select an understandable group of names. The work of Bookman
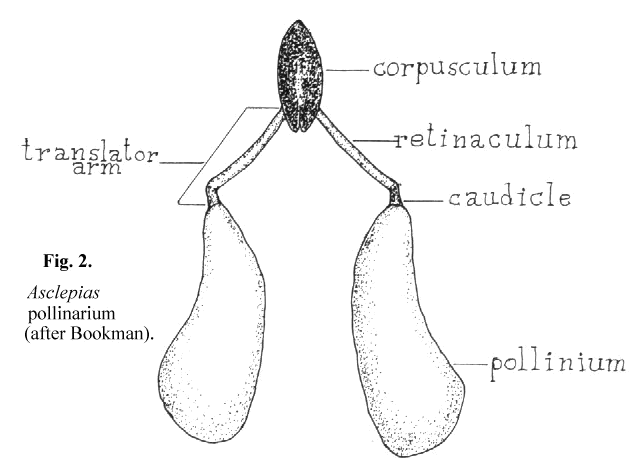
4 seems to have been widely accepted by writers dealing with the succulent asclepiads. This may not be appropriate since her paper dealt with Asciepias speciosa, a non- succulent asclepiad in which the mechanics of pollinium insertion is somewhat different from that of the stapeliads. Her paper also includes an extensive review of earlier writings on pollinarium terminology. According to Bookman, as can be seen in Figure 2, the pollinarium consists of two pollen sacs, the pollinia, each attached to a caudicle which in turn is attached to the retinaculum. The caudicle and retinaculum are each parts of the translator arm. Translator arms from each side are attached to the central structure, the corpusculum. The corpusculum and both translator arms are collectively called the translator apparatus.
The very complete report of Schill and Jakel5 deals with 114 of the asclepiad genera utilizing both scanning electron microscopy and light microscopy. They have also used the term pollinarium for the entire pollinating structure. In their paper, the central structure is called the "klemmkörper" which they translate as 'corpuscle.' The structures joining it to the pollinia are called caudicles. They describe four basic types of caudicle which they have named the one part filiform, the two part filiform, the winged and the flat.
Schnepf, Witzig and Schill6 have shown, by the use of electron microscopy, that the translator apparatus is formed as a secretion product of the underlying cells. The entire structure, the pollinarium, consists of a translator apparatus and the two attached pollinia. Since Bookman's terminology is based on Asclepias, some interpretation is needed to use the same terms on the somewhat different pollinaria of the stapeliads. In Figure 3 a representative stapelia pollinarium is shown. It will be noted that the caudicle which connects the pollinium to the rest of the translator apparatus does not connect at the end of that portion which is labelled the retinaculum in Asclepias. In stapeliads there is considerable variation in the manner with which the caudicle is joined to the rest of the translator apparatus. In some cases the attachment takes place in the midportion of the caudicle wing while in others it attaches directly to the corpusculum. There is also considerable variation in the length and width of the caudicle wing. It would seem to be better to restrict the use of the term retinaculum to those asclepiad species where the structure resembles that of Asclepias. In the stapeliads the structures attached to the corpusculum are better named the caudicle wing and the caudicle. The two caudicles and the corpusculum are referred to as the translator apparatus.
It is not clear from Bookman's paper whether she believes that the pollen tubes emerge from the concave or the convex side of the pollinium. In her text she states that 'the pollen tubes emerge from the more curved edge' and then describes another author's finding of notches in the wall of the convex edge where the pollen tubes first emerge. Her diagram shows the pollen tubes emerging from the concave edge. Chaturvedi7 shows germination of Cynanchium pollinia in vitro to take place on the edge away from the center of the pollinarium. It would seem likely from the structure of Asclepias that pollen tubes emerge from its outer, concave edge. There is no structure on the Asclepias pollinium that is specifically adapted to attachment to the receptive area since in this type of pollinium the entire structure is pulled into the receptive area which is described as the opening between the anther wings.
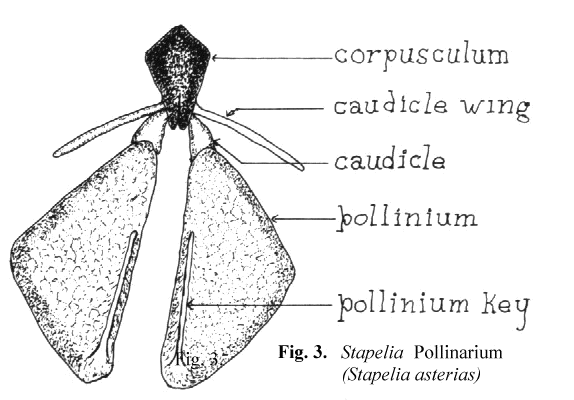 In the stapeliads a different mechanism of pollinium attachment is observed. Stapeliad pollinia, when viewed in situ, have a minute rod shaped structure broadly attached
to the medial, superior surface of the pollinium. This structure has been named the anchor margin or wave crest by other authors. When pollination occurs. this rod like
structure is inserted into the receptive area in a key and lock fashion. For this reason, I have preferred to refer to it as the pollinium key. This can be seen in Figure 3.
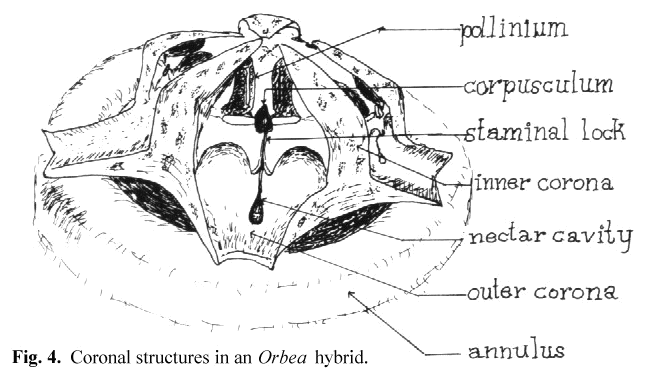
The receptive area varies in form between the different genera of stapeliads but follows the general pattern of a slot which is open toward the base of the flower and
narrows toward the top. In Huernia and Duvalia this slot is relatively wide while in most other genera it is narrow. Other authors have referred to this slot
as the guide rails or the anther wings. In keeping with the functional approach to the key and lock insertion mechanism, I have preferred to call this slot the staminal lock
(see Fig 4). For simplicity I will refer to these structures as the key and lock in the remainder of this paper. Thus we can see that the stapeliads differ from Asclepias
in having only the key inserted into the lock rather than having the entire pollinium inserted between the anther wings.
In the stapeliads a different mechanism of pollinium attachment is observed. Stapeliad pollinia, when viewed in situ, have a minute rod shaped structure broadly attached
to the medial, superior surface of the pollinium. This structure has been named the anchor margin or wave crest by other authors. When pollination occurs. this rod like
structure is inserted into the receptive area in a key and lock fashion. For this reason, I have preferred to refer to it as the pollinium key. This can be seen in Figure 3.
The receptive area varies in form between the different genera of stapeliads but follows the general pattern of a slot which is open toward the base of the flower and
narrows toward the top. In Huernia and Duvalia this slot is relatively wide while in most other genera it is narrow. Other authors have referred to this slot
as the guide rails or the anther wings. In keeping with the functional approach to the key and lock insertion mechanism, I have preferred to call this slot the staminal lock
(see Fig 4). For simplicity I will refer to these structures as the key and lock in the remainder of this paper. Thus we can see that the stapeliads differ from Asclepias
in having only the key inserted into the lock rather than having the entire pollinium inserted between the anther wings.
I will return now to the method of pollinarium removal and pollinium attachment. These plants are dependent on pollinating insects for the completion of natural pollination.
Most of them are pollinated by carrion flies and small gnats which are attracted by the carrion colors and odors. When the insect approaches the flower it attempts to reach
the nectar or to place its ovipositor on the flower to lay its eggs. In doing this a portion of the insect often becomes caught in the lock and in the process of disengaging
itself the trapped portion is drawn upward into the groove in the corpusculum. which is a continuation of the grooved lock. The insect then continues its effort to free itself
and in doing this it pulls the entire pollinarium from its attachments. Plates #1 and 2 show a fly trapped by the corpusculum of Stapelia flavopurpurea. In this
situation, the fly was trapped. but was not strong enough to remove the pollinarium.
After observing a number of trapped flies. I have noted that the structure which is usually trapped is a small bristle on the leg or proboscis. The entire leg or proboscis presents too large an object to pass into the narrow groove of the lock and the corpusculum. Perhaps in the smaller gnats this may not be the case. I have noted in artificial pollination that the surface of the pollinium which lies away from its attachment before removal is the one which must face the lock if insertion is to be accomplished. This is true because of the angling of the key to that side. I believe it is the need for this orientation that explains the function of the inner and outer coronas. These structures tend to restrict the direction of entry of the pollinator so that the pollinarium is removed and oriented on the insect in a manner which favors insertion when the insect visits the next flower. This appears to be more critical in some genera than in others. In Hoodia there are no central orienting structures and the orientation of the pollinarium appears to be a matter of chance.
In visiting the next flower, pollination occurs when the pollinium key is trapped at the opening of the staminal lock and pulled into the lock when the insect struggles to free itself. Frequently the pollinarium will break at the caudicle and the insect will leave with the remaining translator mechanism and the other pollinium. It is possible that this assembly will pollinate the next flower that is visited by the insect.
Although field hybridization is known to occur. it is relatively infrequent. There are several factors which appear to be responsible for this. It has been observed that pollinators are quite specific in limiting themselves to a single stapeliad species in areas where several are found in the same region. Mechanical differences are also limiting factors in the field. A key of excessive size will not fit into a smaller lock. Duvalias tend to have a large lock and key. Although Duvalia and Piaranthus may be found in the same habitat, they rarely hvbridize. Insertion of the large Duvalia key is prevented by the small Piaranthus lock and the reverse cross is unlikely because the small Piaranthus key slips through the large Duvalia lock. In the laboratory, mechanical manipulations make it possible to place pollinia and produce hybrids that would not occur in nature. There are habitats where mechanically compatible species are present. Huernia vereckeri and Huernia longituba ssp. cashelensis grow side by side in Zimbabwe and numerous intermediate hvbrids are seen in the region. The production of a great many intergeneric hybrids by artificial pollination suggests that there are limited genetic incompatibilities in the group. The limitation of field hybridization seems primarily a factor of geographic isolation, pollinator specificity, and mechanical barriers.
Observations of pollinia germinated in glucose solutions have shown that the point of emergenee of the pollen tubes is always the key. In these solutions, this structure swells and forms a well defined pore through which the pollen tubes emerge (see Plate 5). If one attempts to remove a pollinium on the day after insertion into the lock, it will be found to be very tightly held by the enlargement of the key and the developing pollen tubes. I believe that this swelling of the key, which occurs with the absorption of fluid, holds the pollinium in place and keeps it from being pushed away by the force of the mass of germinating pollen tubes.
Having reviewed natural pollination, let us now consider artificial means of pollination. Although it may be possible to develop the skill required to insert the pollinia of the larger flowered varieties with the help of a hand lens, I would consider that a very limiting approach.
Perhaps a visor type binocular magnifier would allow some pollinations to be done. I have found that a dissecting microscope is a very necessary aid in this work. I use a Bausch and Lomb Stereozoom instrument which allows magnification from 10 x to 70 x. Most manipulations are done at 20 x although the smaller flowers may require 40 x to 50x. The instrument is mounted on an industrial mount which allows it to be placed over a living potted plant to permit the manipulations required for pollination. At the beginning of my efforts I considered obtaining a micromanipulator and consulted a supplier of these instruments. I was advised that they would have too small a range of motion for my needs, but in the course of the discussion I learned that the tools used in these instruments were generally very fine microneedles made by pulling heated glass rods. I made a series of hooks and needles from drawn glass and found them very useful in manipulating pollinia. Unfortunately they were much too delicate for this sort of work since every time I used them they very quickly snapped. A search for a more reliable micro hook led to the discovery of a very fine #6-0 stainless steel wire that was being used for eye surgery at the time. Several different hooks were made with the help of the microscope. These were mounted in pin vises and have remained the most important instruments for the placement of the smaller pollinia. These hooks are usually too soft for the removal of pollinia but they are perfect for orientation of pollinia and seating in the lock. A #7 watchmakers forceps is the other tool that I have found extremely useful. These forceps are usually not quite fine enough when received but can be sharpened on a very fine hard stone while watching under the microscope. It is essential that the alignment of the tips be maintained. The forceps are used to dislodge the pollinarium and remove it from the flower. Pollinaria are placed on a clean sheet of white paper and labelled by drawing a circle on the paper with the name of the plant in the circle. When several plants are being used in a working session, the pollinaria are removed and placed on this paper as each plant is brought to the microscope. The crosses are made by taking a pollinarium from the paper to the new flower with the forceps and laying it on the flower. Several blocks or bean bags can be used to steady the working hand. All movements under the microscope must be very fine finger movements. An attempt to work without supporting the hand would result in movements that are too coarse to allow pollination in all but the larger flowers. The pollinium is then positioned with the wire hook and the key is pulled into the lock. In some cases it is necessary to do the final seating of the pollinium with the forceps. I prefer to pull the pollinium into the lock as far as possible. There are occasions when one is inserting small pollinia into large locks that one will pull the pollinium through the top of the lock. With increasing experience the right amount of pull will be found to prevent this situation from occurring.
Some flowers have deep corolla tubes which make access to the pollinia difficult. Usually it is possible to do the manipulation with a longer wire. When this is not possible, the corolla can be dissected away to expose the lock and make pollination easier. Cutting away the corolla results in bleeding of fluid from the cut edges. This can be absorbed with small pieces of soft toilet tissue to keep the fluid from getting in the way of the pollination process. The dissection of the corolla seems to make no difference to the successful pollination as long as the dissection does not extend into the portions which remain after the flower normally separates from the pedicel.
There are some flowers which are supplied with large amounts of nectar. On occasion, the nectar completely covers the lock, making artificial pollination difficult if not impossible. I have found that this is most easily removed by making a small pointed wad of the toilet tissue and touching this to the nectar with the forceps while watching under the microscope. The nectar is quickly absorbed and the pollination can be attempted. One must be careful not to touch the lock with the paper wad as a fiber will often be caught in the lock and result in the removal of a pollinarium which may be needed for pollination. In nature, this excess nectar is probably removed by the insects which visit the flower. In pollinating Piaranthus, I have noted that the nectar is often helpful as the capillary action of the fluid holds the pollinarium to the surface and when it is lifted the key slides into the lock very easily.
As with most natural processes, there are occasions when faults in the the process make pollination impossible. Some flowers will regularly show pollinia that have germinated in situ. These pollinia have a tuft of pollen tubes projecting from the open key and are not usable for pollination. The growth of these pollen tubes appears to be quite random and not directed at the lock in any way. These flowers do not self pollinate from this in situ germination. I have seen this most commonly in Piaranthus and it may be related to the copious nectar mentioned above. On the other hand, Pseudolithos flowers are often drenched with nectar but I have never observed in situ germination in this genus.
Some clones will regularly produce flowers with faulty pollinaria. They may have a normal translator apparatus with useless vestigial pollinia attached. This fault is seen in all flowers produced by this clone. The remainder of the flower is normal and can be fertilized but it cannot produce pollinaria to fertilize another flower. In other cases the caudicle has been found to be deficient such that there is no means to transport the pollinium. When the corpusculum is removed, it comes away without the pollinia attached. As in the other defect, this fault is seen in all flowers produced by this clone.
Once the pollinium has been inserted it begins to absorb fluid from the secretions in the lock and after 8 or 10 hours one can see very distinct swelling of the pollinium. Before insertion, the pollinium has a dark, shiny, glassy appearance. After this swelling has occurred the pollinium becomes lighter, dull and cloudy. One may easily observe pollinium germination by placing a pollinium in the nectar at the base of a flower and observing it under magnification 10 or 12 hours later.
There has been some question as to the course of the pollen tubes from the lock to the entry in the follicles. I have taken paraffin sections of pollinated Piaranthus flowers and found that these tubes penetrate the spongy tissue of the stigma head to reach the follicles which lie immediately beneath it. In other cases I have dissected pollinated flowers of other genera and seen tufts of pollen tubes penetrating the back of the lock to enter the stigma head. In recent dissections of naturally pollinated Asclepias syriaca, I have observed tufts of pollen tubes growing through the space along the lower margin of the stigma head. Apparently both courses of pollen tube growth occur in the asclepiads.
Within two to four days after pollination, the corolla will separate from the pedicel and the sepals will close over the follicles. A pedicel which has not been fertilized may remain attached to the peduncle from a few days to a month. One cannot really be confident of pollination until the pedicel has persisted for at least a month. The emergence of the follicle from a fertile pedicel takes place at a very varying period of time. The shortest period I have observed was eleven days. This was most unusual as the more common timing requires many months or even years! I have seen held fertilized specimens of Caralluma plicatiloba, collected in North Yemen, produce seed pods in each of three successive years. In material that I have artificially pollinated and grown in my greenhouse, those flowers that were fertilized in late summer and fall will usually produce mature seed the following spring. The spring pollinations will often produce ripe seed late in the same spring.
I must caution that pedicel persistence and follicle emergence does not necessarily assure a good result. There are many occasions when wide intergeneric crosses are made, when fully developed follicles will be produced but the seed will not contain embryos. This seed is very thin to the touch and has the appearance of chaff which, of course, will not germinate.
The behavior of the pedicels after successful pollination is variable and quite interesting. In some species the pedicel will show extreme thickening. In others there will be a great elongation raising the follicles high above the plant. In those plants which normally have prostrate pedicels, the pedicels will become erect, again raising the follicles above the plant. This behavior of the pedicels has obvious survival value as it raises the follicle to a level where the seed will be more readily dispersed when the ripe follicle dehisces. In nature the seeds are widely dispersed by the wind. In order to contain the seed so that it is not lost after dehiscence in the greenhouse I have found the use of tubular finger bandage most helpful. This is placed over the mature follicles and will contain the seed so that it can be collected when it ripens.
It is very difficult to keep flies away from flowers in the greenhouse. As a result, most large collections of stapeliads will show frequent fly pollinations. Seeds from these pollinations will often produce interesting hybrids. For my purposes these seeds are usually not used as there are more than enough tagged pollinations to grow. In all of the artificial pollinations, the plants are protected by keeping them in an area which is protected from flies or by pollinating all five locks so that fly pollination cannot take place. All pollinations are numbered and recorded so that reliable data can be accumulated.
If the cultural problems facing the stapelia grower were completely solved, progress in obtaining data on self and cross fertility would be much faster. Unfortunately many successful pollinations are lost to disease during the difficult winter months. This has made the accumulation of data on a large scale less reliable. During a period of good growth and limited disease damage, data were collected on a number of pollinations. These data are shown in Figure 5. It can be seen that there is a wide variation in the degree of self fertility in the different genera. Duvalia, Orbea and Stapelianthus have shown the greatest degree of self fertility. Most of the genera studied have shown at least some self fertility. Fertility in all genera is far greater when interclonal crosses are made between members of the same species. Surprisingly, there is a great deal of fertility observed in crosses between different species and between plants of different genera. It is of interest to note that most crosses result in hybrids that produce plants which are intermediate between the parents. In cases where one parent was a polyploid the offspring all have had the appearance of the polyploid parent. This has been noted in using Orbea woodii (2n = 44) in the cross. In most stapeliads, a chromosome number of 2n = 22 has been reported.
It has been noted that, in some intergeneric crosses, the offspring have had defects in the production of chlorophyll. The seedlings may be variegated and on occasion have no chlorophyll at all. In the latter case the seedlings die soon after germination. When there is some chlorophyll the plants eventual grow out of the variegation. On occasion a hybrid has been produced where the chlorophyll lack is persistent and a light gray green stem color persists.
It is my hope that these observations will allow other interested growers to pollinate this unusual group of plants. Some time is needed to develop the skills that are required. A large dose of patience is recommended.
| Results of crosses | ||||||||
|---|---|---|---|---|---|---|---|---|
| GENUS | Selfed | Interclonal Cross | Interspecies Cross | Intergeneric Cross | ||||
| Total done | Percent fertile | Total done | Percent fertile |
Total done | Percent fertile | Total done | Percent fertile | |
| Caralluma | 154 | 8% | 25 | 56% | 3 | 67% | 10 | 10% |
| Duvalia | 37 | 22% | 5 | 100% | 5 | 40% | 9 | 33% |
| Echidnopsis | 8 | 0% | 6 | 17% | --- | --- | 1 | 0% |
| Frerea | 19 | 0% | 5 | 80% | --- | --- | 24 | 13% |
| Huernia | 70 | 6% | 2 | 100% | 18 | 28% | 12 | 50% |
| Orbea | 21 | 29% | --- | --- | 34 | 44% | 16 | 17% |
| Piaranthus | 132 | 7% | 18 | 28% | 8 | 100% | --- | --- |
| Stapelia | 52 | 4% | 13 | 62% | 14 | 14% | 22 | 19% |
| Stapelianthus | 8 | 38% | --- | --- | 4 | 100% | --- | --- |
| Tridenta | 16 | 6% | 12 | 58% | 11 | 18% | 6 | 50% |
For her patience. understanding and encouragement during the many hours spent isolated over my microscope and camera, I would like to thank my wife, Beatrice.
| 1. | Newton, L. E. 1984. Terminology of structures associated with pollinia of the Asclepiadaceae. Taxon 33(4): 619-621. |
| 2. | Harold, K. 1985. The worth of pollinarium. Asklepios 35:29. |
| 3. | Bruyns, P. V. 1985. Pollinaria and Ceropegia arabica and allies. Asklepios 36:65-67. |
| 4. | Bookman, S. S. 1981. The floral morphology of Asclepias speciosa (Asclepiadaceae) in relation to pollination and a clarification in terminology for the genus. Amer. J. Bol. 68(5):675-679. |
| 5. | Schill. R., and Jakel, U. 1978. Beitrag zur Kenninis der Asclepiadaceen-Pollinarien. Tropische und subtropische Pflanzenwelt 22. |
| 6. | Schnepf E., Witzig, F., and Schill, R. 1979. Uber Bildung und Feinstruktur des Translators der Pollinarien von Asclepias curassavica und Gomphocarpus fruticosus. Tropische und subtropische Pflanzenwelt 25. |
| 7. | Chaturvedi, S. K. 1987. Pollination and pollen germination in Cynanchium canescens, Asklepios 40:93-96. |